Studying the chemistry of cloud formation at CERN
Lauren Smith
Jan 16, 2025
Source: Ali Stinchfield
Neil Donahue has long loved physics. He has focused his career, however, on concerns about the state of the environment. When he chose to train in meteorology and atmospheric chemistry, he thought he was leaving particle physics behind. "The irony is that here I am, doing particle physics at CERN," Donahue says, clarifying that the atmospheric particles he works with are much, much bigger than the elementary particles for which CERN is commonly known, like the Higgs boson.
Donahue, Thomas Lord University Professor of chemistry, chemical engineering, and engineering and public policy, is part of the Cosmics Leaving Outdoor Droplets (CLOUD) experiment at CERN. The goal of the interdisciplinary collaboration is to understand the chemistry and physics that lead to the formation of small molecular clusters that then grow into particles in the atmosphere.
The concentration of atmospheric particles affects the number of droplets in clouds. Clouds with more droplets are whiter. Whiter clouds reflect more solar radiation and can have a cooling effect on the planet, potentially masking some of the warming effect of greenhouse gases.
The connection between particles and clouds is one of the largest uncertainties left in our understanding of climate change. "We need to understand what has happened since the preindustrial era and also be able to inform policy as we go forward," says Donahue. CLOUD scientists from 17 institutes in nine countries are establishing the experimental foundation to understand what clouds were like back in time, before scientists could measure them, and to predict what clouds will be like as climate changes due to human influence.
We need to understand what has happened since the preindustrial era and also be able to inform policy as we go forward.
Neil Donahue, Thomas Lord University Professor, Chemical Engineering, Chemistry, and Engineering and Public Policy
The CLOUD consortium recently identified a new mechanism for atmospheric particle formation, driven by isoprene. Isoprene is emitted by trees. The CLOUD findings, published in Nature, imply that biogenic particles were more abundant in the preindustrial atmosphere than previously thought.
Donahue's core research interest is the way that organic compounds participate in making and growing a molecular cluster. Atmospheric particles form out of the air. One molecule sticks to another molecule, then another and another.
A cluster of molecules starts at about a nanometer in size and has to grow to about a hundred nanometers in order to affect clouds and climate. "The story of growth for particles, especially in the first one to ten nanometers, is really important because most of them die," Donahue says.
Small molecules tend to be volatile, or not very sticky. Donahue is working to answer two closely-related questions: how sticky are organic compounds, especially to really small particles, and what was the chemistry that made the sticky molecules in the gas phase?
The sticky molecules in organic compounds in the atmosphere don't come from emissions. They are created when a precursor molecule is oxidized in the atmosphere. Donahue is particularly interested in trying to understand that chemistry.
CERN offers unique capabilities to create and control the experimental conditions he needs. For example, an electric charge (ions) in the atmosphere increases the rate of particle formation. Most of the ions in the atmosphere come from cosmic rays. With CERN's Proton Synchrotron, scientists can make high-energy elementary particles, like those in cosmic rays. They can vary abundance and ionization to replicate how the rate of cosmic rays varies with time and altitude.

Source: CERN
The CLOUD experiment at CERN
The cylindrical chamber used in the CLOUD experiment is 27 cubic meters, and researchers must manipulate conditions at large scale while being incredibly precise. While at CERN for six weeks this fall, Ali Stinchfield took shifts running experiments with other researchers and changing various conditions in the chamber. "We can vary the temperature through the full range that the atmosphere sees, -60 degrees centigrade up to 30 degrees centigrade. We can control the humidity from bone dry to over a hundred percent," says Donahue.
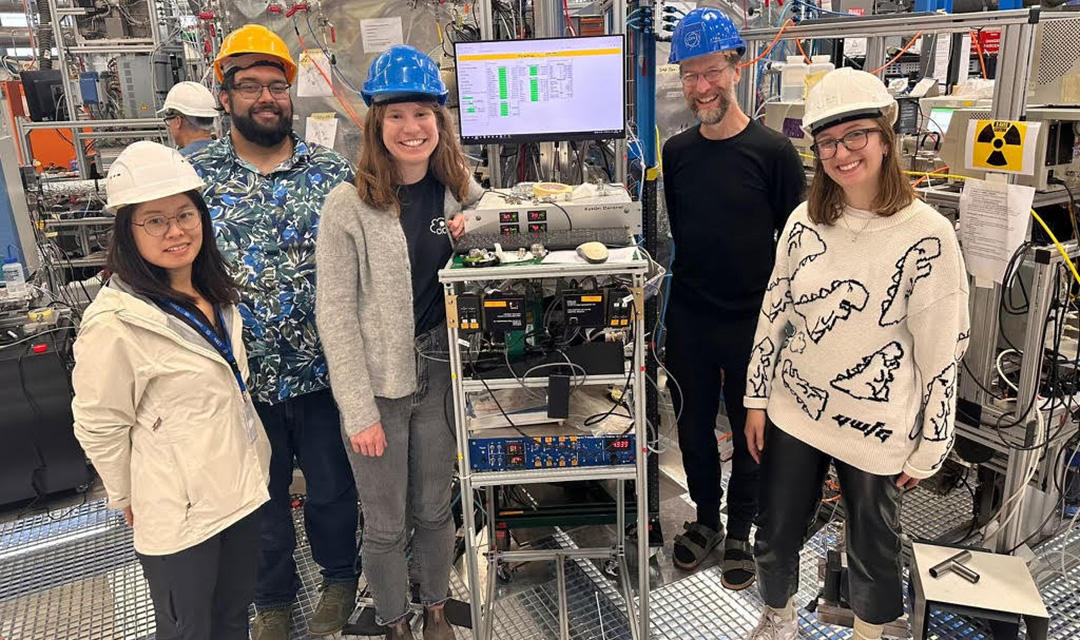
Stinchfield, a Ph.D. student in the Department of Chemical Engineering, along with Jenna De Vivo and Nirvan Bhattacharyya from the Donahue group, also worked on a specialized mass spectrometer that the group adapted for the CLOUD chamber. It collects both gas and particle measurements that help researchers identify and understand their composition.
Donahue's research group typically travels to CERN every autumn. "There's a palpable excitement and energy because everything we're doing pushes the boundaries of atmospheric science daily," says Stinchfield. "Research at CERN is driven by learning, exploration, and a profound sense of curiosity."
For media inquiries, please contact Lauren Smith at lsmith2@andrew.cmu.edu.
Pictured, top: The Donahue group around their specialized mass spectrometer, which is attached to the CLOUD chamber behind them. From left: Han Ding (member of the Gordon Group), Nirvan Bhattacharyya, Jenna De Vivo, Neil Donahue, and Ali Stinchfield.
