Student spotlight: Robert Burnley
Lauren Smith
Jul 25, 2024

Source: Robert Burnley
Robert Burnley kayaking past downtown Pittsburgh
With skates and sneakers, Robert Burnley is training for the next Berlin Marathon. In September, he'll complete the inline skating marathon, which he has done twice before. Then, about 12 hours later, he'll run it for his first time.
Burnley has been an inline skater since he was a kid. As soon as he moved to Pittsburgh for the chemical engineering Ph.D. program at Carnegie Mellon University, he started skating in nearby parks. A passing runner told him to look into the Berlin Marathon. "It was like he swooped in to tell me to do this thing," says Burnley, who has never seen the person again to thank him. "I took him up on it, and now I'm planning to do it every year until I can't skate anymore."
To inspire others to be active, enjoy life, and explore the world, Burnley started using a drone to take photos and videos while skating and while kayaking on local rivers. "Don't just stay in your little bubble," he says. "You're going to miss out on everything if you do."

Source: Robert Burnley
The Monongahela River
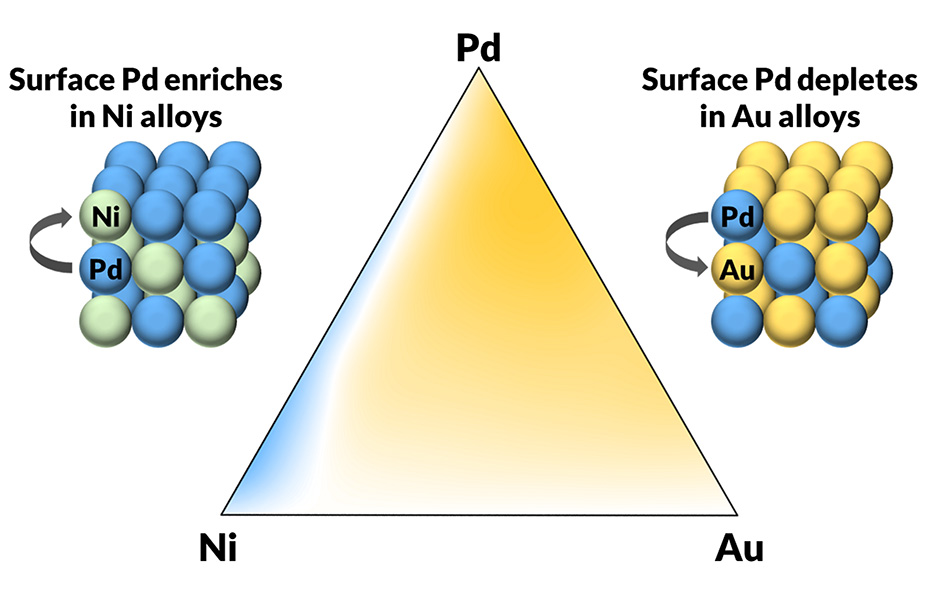
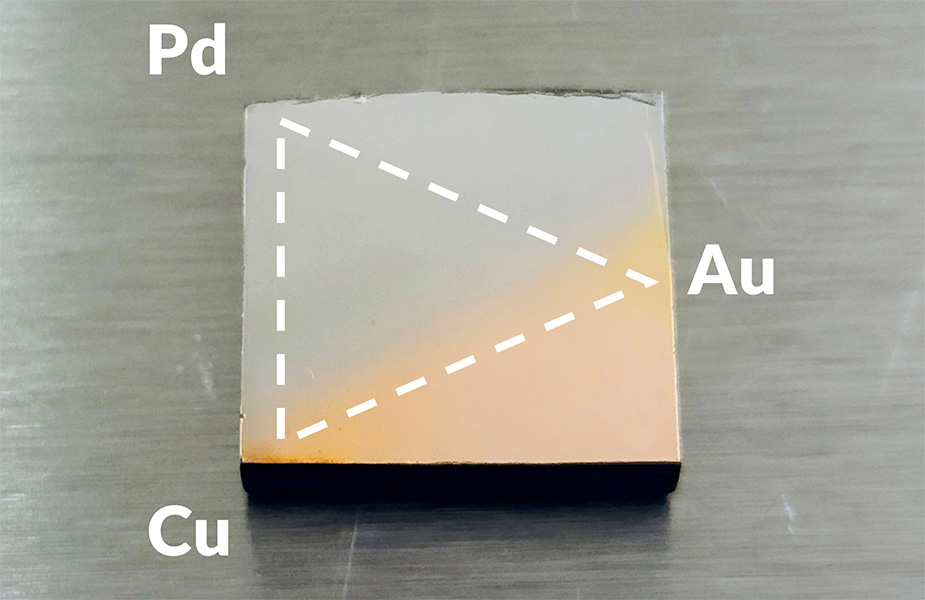
Whether traveling to Berlin for the marathons, paddling freely on Pittsburgh's rivers, or experimenting in the lab, Burnley finds moments of beauty. His research is focused on surface segregation in alloys. When metals are mixed to create an alloy, the surface of the resulting alloy often differs from the composition of the mixture. Burnley remembers the first time he watched it happen right in front of him. He says it was beautiful. He created a three-component alloy, then removed the surface layer to reveal the disordered mixture. Next, Burnley rapidly heated the alloy and then held the temperature constant. He watched and measured in real time as the surface of the alloy rearranged itself to create a new equilibrium surface. Repeating the experiment at different temperatures and with different alloy mixtures helped Burnley to understand the thermal stability of alloy surfaces.
Surface segregation can create an entirely unexpected alloy in the surface. "You might think you're mixing a 50-50," says Burnley, "but you're getting a 20-80 surface." The problem is compounded by the addition of more materials. Burnley's advisor, Andrew Gellman, specializes in ternary alloys.
The Gellman Research Group has a unique high-throughput methodology. They create thin films with laterally varying compositions. "We can essentially create all the possible alloys in the form of this film and allow them to create their equilibrium surfaces," explains Burnley. Researchers deposit the film onto a substrate and measure the surfaces. Pairing the thin film technology with a microreactor array, they are able to do chemical reactions across a wide range of alloy compositions.
"Suppose the literature shows that a binary alloy might be improved by substituting a small amount, or perhaps a significant amount, of a third component. We can make a film which contains all the possibilities of those catalyst compositions and then do the reactions right on that surface and map the reactivity to the composition space," explains Burnley.
Because surface segregation is not well understood, Burnley set out to create large data sets of physical measurements. The data sets can be used to predict which surfaces are going to be stable.
Burnley's research has implications for catalyst design. Catalysts are very commonly used across industries, from automotive to pharmaceutical to energy, in the synthesis of compounds we use every day. The design process to find optimal catalysts for a given chemical process involves expansive sampling. Large libraries of experimental data, like those Burnley is creating, can be used to benchmark work with quantum models and artificial intelligence. "If you can design a catalyst that's even marginally better than the state-of-the-art catalyst, you generate massive savings, and you might open up pathways that were previously not economically feasible," says Burnley. This includes carbon utilization and hydrogen power.
If you can design a catalyst that's even marginally better than the state-of-the-art catalyst, you generate massive savings.
Robert Burnley, Ph.D. student, Chemical Engineering
Some of Burnley's recent work involves palladium and platinum alloys. Both palladium and platinum are widely used as hydrogenation catalysts. By adding other metals to these catalysts, Burnley hopes to improve their performance. He is continuing his experiments on these alloys to understand how the crystal phase affects the stability of the surface.
After completing his Ph.D., Burnley will return to the Chicago area, where he has a large family. He is considering research roles in academia or national labs, as well as a return to his electroplating roots in the automotive industry, where he worked after high school.